We make rapid testing universal
Discover how to boost your diagnostic business

Qassay®: The Lateral Flow Reader of the Future
Qassay® Lateral Flow Readers are designed to assist our partners in assay development and manufacturers of lateral flow test strips
We are on a mission to universalize rapid testing. Thanks to our innovative technology, Qassay enables both qualitative and quantitative results.
Best in class sensitivity to detect low analyte detection levels
Easy to use proprietary analysis software and tools to quantify test based on Cloud machine learning and analytics
Faster than ever before. 5 second read time.
Wide dynamic range that covers the analytes’ concentration range of interest.
Full access to raw data (test strip images, CSV and Excel) to support data analysis and research needs
End-to-end ecosystem
Unlock the potential of your diagnostic business
End-to-end ecosystem: composed of state-of-the-art electronic portable reader (colorimetric & immunofluorescence), mobile application and highly scalable cloud software.
Based on advanced multi-spectral sensor technology combined with Math Models, Qassay® readers are flexible enough to host any type of lateral flow test, ensuring the highest accuracy, sensitivity, and repeatability.
There are no moving parts in the reusable reader, reducing the cost and complexity of the device. It is also suitable for qualitative and quantitative applications and a variety of assay detector chemistries.
Math Models & Algorithms
Faster. Accurate. Easy
Mathematical processing algorithms for a simple manual extraction experience: robust algorithms for the detection and quantification of test and control lines. Strip-wise trained, semi-quantitative and quantitative multi-analyte assay evaluators.
After a short period of training (proprietary technology), and a specific math-programming we are able to generate a model capable of quantifying and qualifying results by line in a single cassette. If you are a manufacturer, request a demo with your own bio-markers.
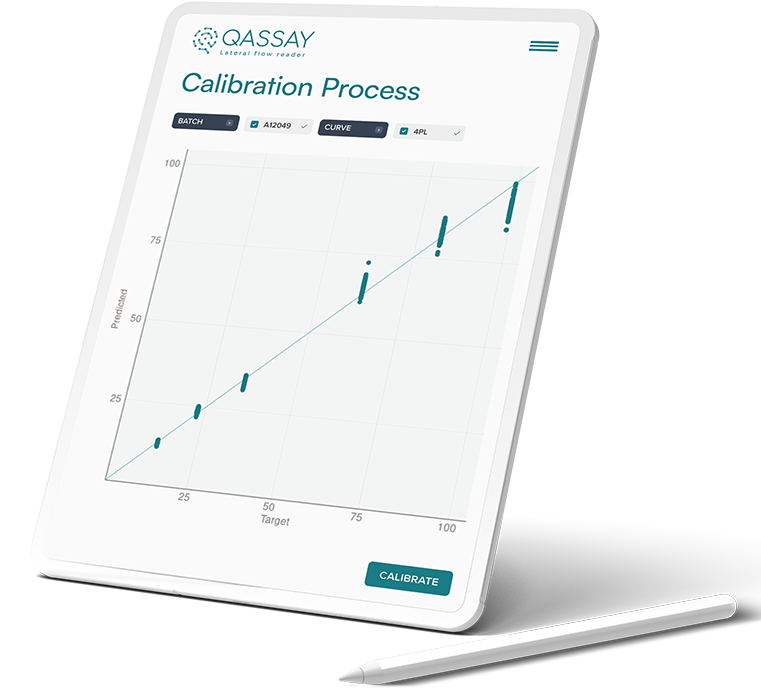
Customer portal
Always with you
Our reusable reader platform provides our partners with an affordable solution for POC applications needing high-precision diagnostics. Qassay® has extensive in-house data security and patient privacy expertise and provides a full range of data management options, cybersecure and with state of the art of encryption methods.

Cloud & Data Management
Our proprietary cloud platform utilizes industry leading cloud infrastructure to provide secure storage and access to data. This translates into real value for our partners by mitigating the burden of infrastructure or regulatory implications.
Secure REST API
Extreme compliance with cybersecurity and open architecture to other services and systems: Integration with 3rd parties, Laboratories, Hospital Information System and HL7 protocol.
Prevalence maps
No more epidemics
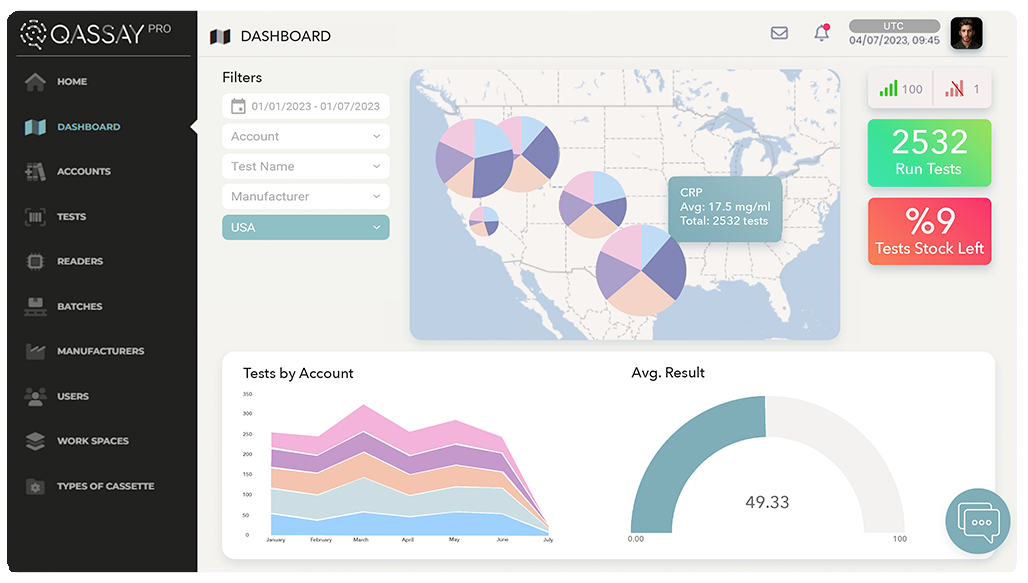
Qassay® platform offers the best mapping capabilities in order to ease Institutions prevalency map analysis procedures for quick reaction to an epidemic threat.
Now it’s your turn
Tell us about your next challenge. We’ll make it possible
You ask, we answer
FAQs
1. How does the reader work?
The reader measures light intensity through the combination of LEDs and a spectrometric sensor. The smartphone app acts as a User Interface, guiding the user in taking the correct steps. Data is sent to the mobile app and then to the cloud, where our mathematical models process the data and return a result.
2. Why manual extraction?
Using a manual extraction method, rather than a motorized extraction, helps the device to be cost-effective, while maintaining the repeatibility and sensitivity of a motorized device. The raw data captured by the sensor is processed by our algorithms so that speed is not an influential variable.
3. How can I test the reader?
Before testing the reader, we need to design an adaptor tray for each cassette. Once the cassette is designed and 3D-printed, there are 2 choices to proceed with calibration. 1. Sending a 100 tests with positive control for callibration and an independent validation at a 3rd party lab. This procedure is official and the results can be used for regulatory purposes. 2. If samples can´t be sent to Qassay, a few sample readers will be sent for a remote callibration to your facilites. Complete the form to contact our sales team.
4. Which functionalities does the cloud platform have?
Qassay´s cloud platform enables companies to manage their devices, tests, batches and users more efficiently. The platform is built on AWS and it has a REST API to integrate with 3rd party applications.
5. Is the reader reusable?
The reader is fully reusable, as it has a USB-C rechargeable battery.
6. Which is the regulatory status of the Qassay lateral flow reader?
The Qassay Lateral Flow Reader is CE marked under the CE-746 IVDR, as a Class A instrument
1. What is a Lateral Flow test?
A lateral flow test is a simple and rapid diagnostic test used to detect the presence or absence of a target substance, such as a specific antigen or antibody, in a liquid sample. These tests are often used for medical diagnosis, home pregnancy tests, and various other applications. Lateral flow tests are designed to be easy to use and provide quick results, typically within a few minutes.
2. Why use a Lateral Flow Reader?
Many lateral flow tests, require an instrument to provide a quantitative measurement. Other tests, while qualitative, improve their usability when using an instrument, so that there is no effort from the user for line recognition. Moreover, digitizing results of these tests can make a difference in the remote monitoring of chronic patients or epidemics.
3. What can be measured with Qassay?
There is a wide range of biomarkers that can be measured with Qassay, from cardiovascular diseases to lifestyle markers such as Vitamin D, Ferritin and various hormones. Other notable applications are infectious diseases, metabolic diseases, fertility and much more.
4. Which functionalities does the cloud platform have?
Qassay´s cloud platform enables companies to manage their devices, tests, batches and users more efficiently. The platform is built on AWS and it has a REST API to integrate with 3rd party applications.
5. Is the reader reusable?
The reader is fully reusable, as it has a USB-C rechargeable battery.
6. Which is the regulatory status of the Qassay lateral flow reader?
The Qassay Lateral Flow Reader is CE marked under the CE-IVDR, as a Class A instrument


