The med-tech company is revolutionizing healthcare with its lateral flow reader and begins the process of mass production and commercialization.
Qassay has identified the US market as a priority for its growth
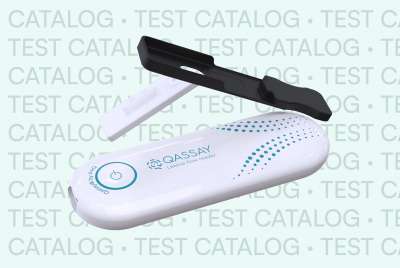
Qassay, the brand specializing in lateral flow readers, has announced the mass production of its medical devices at its facility in Alonsotegi (Spain).
Qassay has developed an innovative lateral flow reader capable of qualifying and quantifying rapid test results. The applied technology combines advanced electronic manufacturing, multispectral sensors with mathematical processing algorithms, as well as a mobile application and cloud-based software. This digital health ecosystem will significantly reduce waiting times for medical test results. With this technology, biomarkers for cardiovascular diseases, sexually transmitted infections, fertility issues, or vitamin levels, among others, can be analyzed.

In recent months, the functionality of the lateral flow reader has been improved, and the production facility in Alonsotegi has been optimized to provide specific workspaces that meet the highest standards of medical regulations applicable to medical devices.
This solution has been approved by AEMPS “The Spanish Agency of Medicines and Medical Devices” and has recently obtained the CE marking necessary for its commercialization in the European Union. Qassay is currently in the process of internationalization, with a focus on the United States due to its high growth potential market.
“With the announcement of mass production, we embark on an exciting phase for Qassay that will allow us to scale our project. We are closer to the universalization of rapid tests,” says Gorka Alapont, Business Development Manager at Qassay.
Related posts:
Frequently Asked Questions about a Lateral Flow Reader